Figure 1. Colour fundus photograph of the left eye demonstrates florid neovascularization temporal to the macula. There are white areas representing fibrosis. No macula oedema was evident clinically.
A 35-year-old lady was referred by her optometrist with diabetic retinopathy.
A 35-year-old lady was referred by her optometrist with diabetic retinopathy. She had been diagnosed at the age of 5 with type 1 diabetes and had been treated with insulin since then. She did not have any visual complaints and her ophthalmic history was otherwise unremarkable. She had not had retinopathy screening for at least two years and was unaware of retinopathy being detected at previous reviews. She had not seen her endocrinologist for a number of years. Aside from Novorapid and Protophane insulin, she was not taking any regular medications. She smoked half a packet of cigarettes per day.
Best correct visual acuities were 6/6 in the right (OD) eye (-0.50/-0.75×100°) and 6/6- in the left (OS) eye (-1.25/-0.25×75°). The anterior segment examination was unremarkable, with clear lenses and no iris or angle neovascularisation. Intraocular pressures were 16mmHg OD and 17 mmHg OS.
Fundus examination of the left eye demonstrated wide areas of neovascularisation elsewhere (NVE), principally temporal to the macula (Figure 1). This was also associated with areas of preretinal fibrosis. There were microaneurysms and some scattered blot haemorrhages in the posterior pole. No neovascularisation of the disc (NVD) was present. No diabetic macula oedema could be visualised clinically with a normal foveal reflex and no hard exudate at the posterior pole. There was no vitreous haemorrhage complicating the neovascularisation.
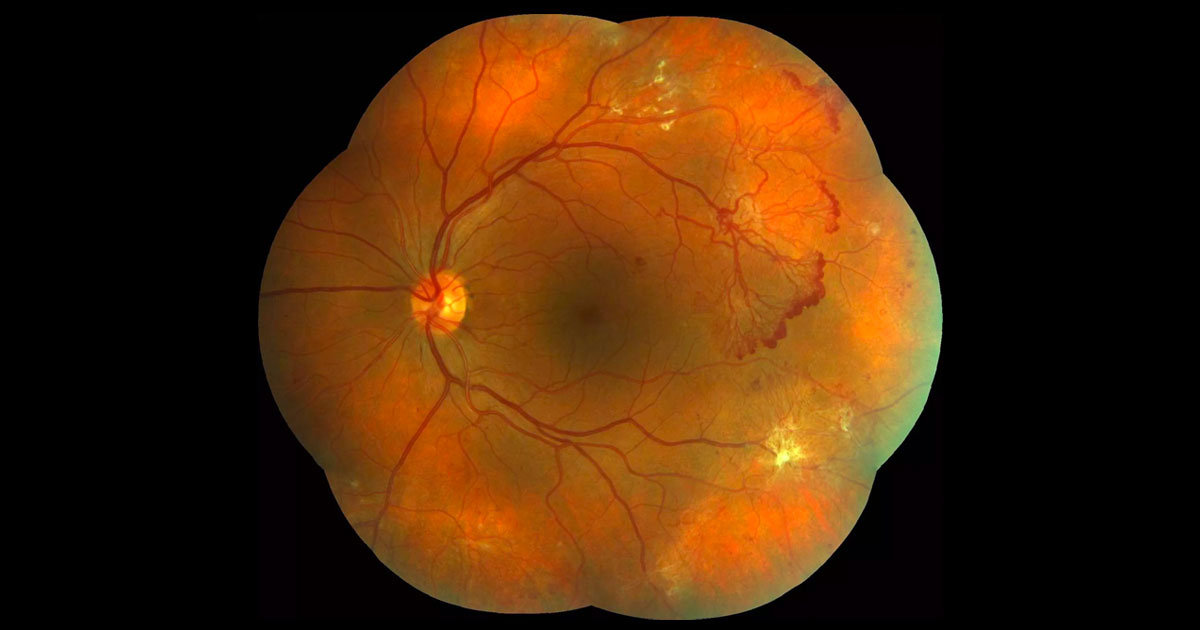
In the right eye there were widespread areas of NVE, principally temporally (Figure 2). Similar to the left eye there was no diabetic macula oedema seen clinically.
In both eyes there were grade 1 chronic hypertensive retinal vascular changes with some AV nipping and arteriolar reflex changes (or “copper wiring”).
Figure 2. Right colour photography demonstrates neovascularization elsewhere and preretinal fibrosis.
There are a number of classification scales for diabetic retinopathy. Retinopathy and diabetic macula oedema (DMO) are graded independently of one another.
The International Clinical Diabetic Retinopathy and Diabetic Macula Oedema Disease Severity Scales(1) are commonly used in Australia:
International Diabetic Retinopathy Disease Severity Scale
| Severity Level |
Clinical Findings |
|
None |
No retinopathy |
|
Mild Non-proliferative diabetic retinopathy (NPDR) |
Microaneurysms |
|
Mod NPDR |
More than just microaneurysms but not severe NPDR |
|
Severe NPDR (4-2-1 rule) |
4 quadrants >20 intraretinal haemorrhages
2 or more quadrants venous beading
1 or more quadrants prominent intra-retinal microvascular abnormality (IRMA) |
|
Proliferative (PDR) |
Neovascularisation
Vitreous/Preretinal Haemorrhage |
International Diabetic Macula Oedema (DMO) Disease Severity Scale
| Severity Level |
Clinical Finding |
|
DMO apparently absent |
No apparent retinal thickening or exudate within posterior pole |
|
DMO apparently present |
Some apparent retinal thickening or exudate within posterior pole |
If DMO is present it can be classified as:
| Severity Level |
Clinical Findings |
|
Mild DMO |
Some thickening or hard exudate within posterior pole but away from macula |
|
Moderate DMO |
Thickening or hard exudate approaching but not involving the central macula |
|
Severe DMO |
Retinal thickening or hard exudate involving the central macula |
In clinical practice optical coherence tomography (OCT) is often used to divide patients into those with centre involving diabetic macular oedema (DMO) and non-centre involving DMO.
On OCT, neither maculae demonstrated any oedema (Figure 3). Fluorescein angiography demonstrated widespread hyperfluorescence arising from areas of neovascularisation (Figure 4). There were extensive areas of peripheral non-perfusion. In both eyes there was irregularity of the normal macula capillary network and some enlargement of the foveal avascular zone consistent with mild macula ischaemia. The patient’s HbA1C was 11.5% (target <7.0%), suggesting poor recent control.
Figure 3. Optical coherence tomography of the left macula does not demonstrate any oedema.
Figure 4. Fundus fluorescein angiography (FFA) demonstrates hyperfluorescence associated with the areas of neovascularization elsewhere. There was no neovascularization of the disc. The foveal avascular zones are irregular as a result of some macular ischaemia.
DIAGNOSIS
Bilateral high risk proliferative diabetic retinopathy without diabetic macula oedema.
The diagnosis was explained to the patient. It was emphasised that although she was asymptomatic there was a high risk of vision loss, even with treatment, on account of the severe retinopathy and retinal ischaemia. The patient was referred back to her endocrinologist who made adjustments to her insulin regime and started an ACE inhibitor for hypertension. She was also convinced to stop smoking. After discussion panretinal photocoagulation laser (PRP) was commenced initially in the left eye. Despite PRP in the right eye the patient presented two months later with acute painless vision loss in the right eye secondary to a large pre-retinal haemorrhage (Figure 5).
Figure 5. Despite panretinal photocoagulation laser treatment in the right eye the patient suffered a large preretinal haemorrhage. Some laser burns can be seen just outside the superior arcade.
The main causes of vision loss from diabetic retinopathy are diabetic macula oedema (DMO) and the complications of proliferative diabetic retinopathy (PDR).
The diagnosis of PDR is made clinically when neovascularisation is seen. These abnormal fragile vessels grow in response to retinal ischaemia. Retinal neovascularisation can occur at the disc (NVD) or elsewhere (NVE). Anterior neovascularisation can develop on the iris (NVI) or drainage angle (NVA), resulting in neovascular glaucoma (NVG). Retinal neovascularisation is prone to bleed, resulting in pre-retinal or vitreous haemorrhage. It may also cause fibrosis and traction that can result in vision loss from epiretinal membrane formation, vitreoretinal traction bands, retinal tears and tractional and/or rhegmatogenous retinal detachment.
Early neovascularisation can be difficult to detect as the vessels are often fine, can blend into the retinal background and only occupy a small patch of retina. Fine NVD can be difficult to distinguish from normal small blood vessels. Normal vessels, however, always taper to an end and do not loop back to the disc.(2) Neovascular fronds always loop back, may form a chaotic network within the loop, and have the top of the loop of wider diameter than the base.(2) On fluorescein angiography neovascularisation will leak, in contrast to normal vessels and intraretinal microvascular abnormalities (IRMA) that do not.(2)
Neovascularisation can be confused with intraretinal microvascular abnormalities (IRMA) seen in severe non proliferative diabetic retinopathy. IRMA appear as tortuous microvascular abnormalities that are thought to be the remnants of vessels resulting from extensive closure of the capillary network.(2) As opposed to neovascularisation, IRMA are intraretinal and not superficial, and appear as tiny and wavy blood vessels lying between retinal arterioles and venules. Neovascular vessels are superficial, often becoming elevated as they become more advanced. They do not run between arterioles and venules and can be seen running across native vessels.
The standard of care for PDR has been PRP laser since the Diabetic Retinopathy Study demonstrated a 50% reduction in severe vision loss with this treatment(3). The laser burns in PRP are applied to the entire retina except for the posterior pole to spare the central visual field. Usually 2-3 treatment sessions are required to complete the laser. Side effects of PRP include peripheral visual field loss, decreased night vision, reduced accommodation and transient exacerbation of DMO.(4)
Recently the Diabetic Retinopathy Clinical Research Network (DRCR.net) published the 2 year results of Protocol S, a randomised trial comparing anti-VEGF monotherapy (ranibizumab) to PRP for PDR.(4) This study found that anti-VEGF monotherapy was non-inferior to PRP in maintaining visual acuity. Anti-VEGF monotherapy was also associated with significant lower rates of vitrectomy, less DMO and better peripheral field sensitivity. The main downside of anti-VEGF monotherapy is the need for ongoing therapy, and the small but serious risk of injection related endophthalmitis.
Extrapolating these results to clinical practice, in patients with both DMO and PDR without traction, most retinal specialists will start anti-VEGF monotherapy and add in PRP after the DMO has stabilised. The decision is more difficult in patients presenting without DMO but with PDR. These patients often have good vision and do not realise the severity of their disease. Approximately 50% of retinal specialists surveyed by the American Society of Retinal Specialists were concerned about patients failing to return for follow up as the main limitation of anti-VEGF treatment over PRP.(5) It is not uncommon for patients to miss appointments particularly if they are required monthly over years. Missed appointments may result in recurrences of active NV and vitreous haemorrhage as the anti-VEGF effect wears off. This is less likely to happen with PRP which has a long duration of effect. At this point in time, most retinal specialists would still recommend patients have PRP over anti-VEGF monotherapy if there is PDR without DMO.
TAKE HOME POINTS
- The principle causes of vision loss in diabetic retinopathy are macular oedema and proliferative retinopathy.
- Proliferative diabetic retinopathy is characterized by retinal neovascularization.
- Neovascularisation can result in vitreous haemorrhage and tractional retinal detachment.
- Panretinal photocoagulation is the standard of care for patient with proliferative retinopathy.
- Anti-VEGF agents may be useful in patients with coexisting macula oedema
- Protocol S has demonstrated anti-VEGF monotherapy to be non-inferior to PRP with further benefits less of peripheral field loss, less diabetic macular oedema and lower rates of vitrectomy.
- Concerns about patients returning for regular injections is a current barrier to anti-VEGF monotherapy for PDR.
REFERENCES
- Royal College of Ophthalmologists. Diabetic Retinopathy Guidelines. London, UK: Royal College of Ophthalmologists ; 2012.
- American Academy of Ophthalmologists Retina/ Vitreous Panel. Preferred Practice Panel Guidelines Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2016
- Diabetic Retinopathy Study Research Group.Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8. Ophthalmology. 1981;88(7):583-600.
- Writing Committee for the Diabetic Retinopathy Clinical Research Network. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy A Randomized Clinical Trial JAMA. 2015;314(20):2137-2146.
- Stone TW, ed. ASRS 2016 Preferences and Trends Membership Survey: Chicago, IL. American Society of Retina Specialists; 2016.
Tags: diabetic retinopathy, neovascularization, macula oedema, vision loss